The water in the pool and water in the glass are at the same temperature. Without going into mathematical detail we can say that thermal energy the energy associated with heatis the average kinetic energy of the particles molecules or atoms in a substance.
Difference Between Temperature And Thermal Energy Difference Between
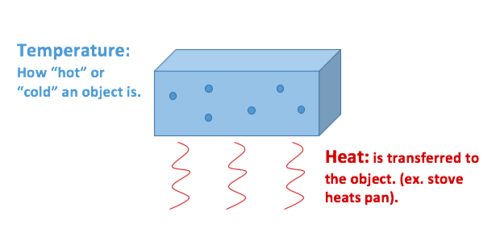
Define heat and temperature and convert between temperature scales.
. In a hot bath thermal energy is transferred from the water to your body. The core difference is that heat deals with thermal energy whereas temperature is more concerned with molecular kinetic energy. Thermal energy measures the total energy of moving particles.
Internal energy refers to the total energy of all the molecules within the object. And Temperature do not mean the same thing. Heat is the transfer of thermal energy whereas temperature is a property the object exhibits.
Thermal energy also known as heat is a form of internal energy of a system. The difference between heat and thermal energy is that thermal energy is not in the process of being transferred. Faster or slower movement of molecules is noticed depending upon the kinetic energy whereas for thermal energy the total number of particles present in an object determines its kinetic energy therefore the bigger.
There exist a fine line which demarcates heat from temperature in the sense that heat is thought of as a form of energy but the temperature is a measure of energy. Heat is thermal energy. Heat on the other hand is energy in transit ie.
B specific heat of the object. Another difference between the two is while heat measures particle movement and potential energy temperature only measures the movements of molecules in an object. The main difference between temperature and thermal energy is that the temperature is not determined by the quantity of the object.
Distinguishing Temperature Heat and Thermal Energy Temperature is related to the kinetic energies of the molecules of a material. In a cold bath thermal energy is transferred from your body to the water. The fundamental difference between heat and temperature is slight but significant heat is the overall energy of the.
Thermal energy is the cause for the temperature of a system. D both a c. Click card to see definition.
Click again to see term. Every system having a temperature above absolute zero has a positive thermal energy. Objective In this activity students will explain the difference between heat and energy as a result of watching the NASA Spotlite video learning the vocabulary collaboratively and discussing how the movement of molecules creates thermal energy.
Faster moving molecules have greater kinetic energies and so the substance has greater thermal energy and thus a higher temperature. Practice Heat Temperature and Thermal Energy Transfer. Students incorrectly think heat and temperature are the same thing.
You both still have a temperature due to the vibrations of your particles but there is no. The thermal energy occurs due to the random movements of the molecules of the system. And changing the temperature of an object therefore means changing the heat energy it contains.
C kinetic energy of the particles in the object. Identify the tools you would need. The hotter the substance the more its molecules vibrate and therefore the higher its thermal energy.
Energy in the process of being transferred from a hotter system towards. Temperature is an average of a distribution of thermal energy - the more energy the higher the temperature but its not always a linear relationship see Latent heat -. Heat and temperature are a closely related topic and as such the difference between the two can be a bit confusing.
And heat is measured in joules while temperature is measured in Fahrenheit Celsius or Kelvin. Temperature is only a measure of energy whereas heat is an energy form on its own. Develop an activity where a hot metal object is place in a container of room temperature water.
The terms are very common due to their wide usage in our day to day life. The temperature of an object is directly proportional to the amount of heat energy it contains. A rise in the temperature of matter makes the particles vibrate faster.
Explain how the motion of the atoms or molecules of a substance affect the temperature of the substance. When this happens there is no longer a flow of energy so no more heat. Difference Between Thermal Energy and Temperature Thermal energy is not a directly measurable quantity whereas temperature is a measurable quantity.
As the temperature of an object rises so does the. Define the main scientific differences between thermal energy heat and temperature by filling in the blanks. Temperature is measured in.
Tap again to see term. Use mathematical computational thinking to compare the differences between thermal energy temperature using system models representing molecules and the energy in an iceberg cup of hot coffee. Thermal energy is what we call energy that comes from the temperature of matter.
Thermal energy is heat. Temperature is the average energy of the matter heat is the transfer of thermal energy from one place to another. Answered 1 year ago Author has 73K answers and 64M answer views.
Heat is the transfer of thermal energy whereas temperature is a property the object exhibits. It is the average kinetic energy of. The temperature of an object can take negative values depending on the unit system used to measure the temperature.
It is an extensive property. The core difference is that heat deals with thermal energy whereas temperature is more concerned with molecular kinetic energy. Explain the difference and the relationship between heat and temperature.
A mass of the object. As the energy is transferred you and the water become the same temperature. External energy is all of the energy outside the ball It is dependent on the motion and position of the ballThe thermal energy is the total kinetic and potential energy of the balls particlesThe temperature is the measure of the internal energy.
It is not in transit but remains as part of the internal energy of the system. This indicates how strong in your memory this concept is. Thermal energy is the total kinetic energy of the substance.
Difference Between Heat And Thermal Energy Difference Between
0 Comments